Effects of Concentration of Polyacrylamide on Salt and Alkali Free Reactive Dyeing of Cotton Fabrics
Authors:
Khadija Akter Hame
Md. Azharul Islam1
Dept. of Textile Engineering
Daffodil International University, Dhaka, Bangladesh
Email: aisumon009@gmail.com1
ABSTRACT
This work was carried out to study the effects of Polyacrylamide concentration on reactive dyeing method of cotton knitted fabrics. Polyacrylamide is a polymer formed from acrylamide subunits. When the cotton fabric is treated with polyacrylamide, the primary hydroxyl groups (-OH) of cellulose is partially modified into amide groups (- CONH2), hence reactive dyes can be dyed on cotton at neutral pH in the absence of electrolyte and alkali. The dyeing method with Polyacrylamide rather than salt and alkali is relatively cheaper and also the environmental impact of this method is eco-friendly. This project was mainly focused on the effects of different concentration of polyacrylamide (5%, 10%, 15%, 20%, and 25% %) on the reactive dyeing method of cotton knitted fabric. The aim of the work was to live the Stitch Length, WPI, CPI, GSM, Color fastness to rubbing and color fastness to Washing of the cotton knitted fabric once the reactive dyeing method. During this investigation, we have a tendency to used three styles of knit materials, as well as single jersey, (2×2) Rib and Plain Interlock to judge the consequences. Once finishing the analysis we have a tendency to found that single jersey, (2×2) rib and Plain interlock materials were showing the amendment in WPI, CPI, GSM, Stitch Length of Polyacrylamide concentration throughout reactive dyeing method. From the treatment we have a tendency to might see that for single jersey, (2×2) Rib and Plain Interlock material, once we were used (15%, 20% and 25%) concentration of Polyacrylamide, this has superb color fastness properties. Currently we are able to say that if the quantity of Polyacrylamide is increase within the instruction the dye affinity of the material additionally increase.
INTRODUCTION
In current apply, cellulosic fibers area unit preponderantly colored with reactive dyes within the presence of a substantial quantity of salt and glued below alkaline conditions. However, dye fixation potency on cellulosic fibers is usually low (varying from 50 – 90%). This leads to an extremely colored dye effluent that is unfavorable on environmental grounds. What is more, the high concentrations (40–100gm/L) of solution and alkali (5–20 gm/L) needed in cellulosic fiber coloring could cause extra effluent issues. Cotton pretreatment before coloring offers an easy and effective technique of up dye-fiber affinity, avoiding the necessity for salt within the dye tub. Cat-ionizing cotton fiber will increase the substantively of anionic dyes thanks to the presence of positive charges imparted to the fiber. Cat-ionization is that the chemical modification of cotton to provide cationic (positively charged) coloring sites in situ of existing radical (-OH) sites. As a result of macromolecule fibers like wool and silk were famous to own sensible dye ability. Coloring cationic cotton leads to larger use of dye and better color values. Additionally, the sturdy dye fiber interactions ensuing from cat-ionized cotton permit coloring with no superimposed electrolytes and smallest rinse and once laundry. However, a fiber with nice dye-attracting properties could still exhibit those attributes in later use and become scavengers throughout washing.
It has been found that pretreatment of cotton before coloring offers an easy and effective technique of rising dye-fiber affinity, avoiding the requirement for salt as solution within the dye tub. It’s been found that’s a physical modifying agent. Its wide selection of properties has found use in chemical action, chelating, liquid activity, and treatment of waste matter, recovery of oil and in polymeric dyes. The aim of this work is to work out the effectiveness of methylamine as a pretreatment agent of cotton materials in rising its dye ability with reactive dyes and in achieving evenness of dye uptake.
1.1 Objectives of the Study
The broad objective of the project work is to learning the effects of concentration of polyacrylamide on salt and alkali free reactive dyeing of cotton fabrics.
The specific objectives of project work are pointed out in bellow:
- To assess the different nature of cotton fabrics after salt and alkali free reactive dyeing, together with (GSM, WPI, CPI and Stitch Length).
- To gain about Polyacrylamide, it’s effects and working process.
- To evaluate the ratting of color fastness to rubbing and washing of on cotton fabric after dyeing with different concentration of Polyacrylamide, also gain the knowledge the environmental effect of Polyacrylamide.
LITERATURE REVIEW
2.1 Salt and Alkali Free Reactive Dyeing on Cotton Fabric
This is a coloring method wherever cotton cloth is colored while not salt and soda. The aim of this coloring is to dye the material while not salt and alkali which is able to be economic, non-toxic and simply process able for the textile process [1].
2.1.1 Requirements of Salt-Alkali Free Reactive Dyeing:
- The conventional technique of coloring of cotton with reactive dyes, alkaline pH is maintained within the dye bathtub. This technique needs higher amounts of electrolytes for exhaustion and alkali for fixation. But, the salt-alkali free reactive dyeing technique eliminates the usage of salt and alkali throughout the coloring method.
- In current apply, cellulose fibers area unit preponderantly artificial with reactive dyes within the presence of a substantial quantity of salt and glued below the alkaline conditions. However, dye fixation potency on cellulosic fibers is mostly low (varying from 55-65%) which ends up during an extremely colored dye effluent, that is unfavorable on environmental grounds. moreover, the high concentrations of electrolytes and alkali needed in cellulose fiber coloring might create extra effluent issues. The salt-alkali free reactive dyeing technique reduces the effluent load of the coloring industries.
2.1.2 Chemistry of Salt-Alkali Free Reactive Dyeing:
To carry out the salt-alkali free reactive dyeing, cotton fiber is changed by a method supported cationic acrylic copolymer. Pre-treatment of cellulosic fiber with compound is believed to supply a chance for increasing each the substantively and reactivity of cellulosic fibers towards reactive dyes beneath neutral conditions. once the cotton material is treated with polyacrylamide, the first group teams (-OH) of cellulose is partly changed into organic compound teams (-CONH2), that intern leads the cellulose to act like as wool fiber and thence reactive dyes are often colored on cotton at neutral pH scale within the absence of electrolyte and alkali. The cationic charged amino teams is also concerned within the surface assimilation of anionic radical of reactive dyes and therefore the dye molecules are connect onto the partially-modified cellulosic substrates by chemical bond formation [3].
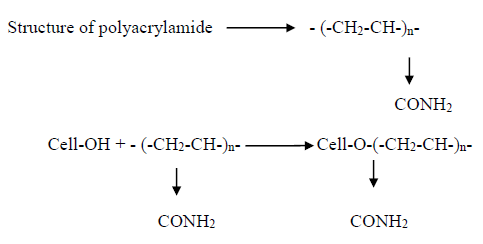
2.2 Polyacrylamide
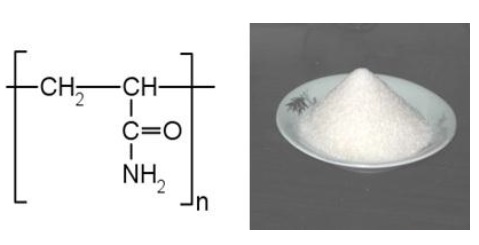
Polyacrylamide may be a compound fashioned from amide subunits. It will be synthesized as a straightforward linear-chain structure or cross-linked. Polyacrylamide isn’t poison. Within the cross-linked kind, the likelihood of the compound being gift is reduced even more. It’s extremely water-absorbent, forming a soft gel once hydrous. Within the straight-chain kind, it’s additionally used as a stuff and suspending agent. A lot of recently, it’s been used as sub dermal filler for aesthetic facial surgery.
2.2.1 Uses of Polyacrylamide
One of the most important uses for polyacrylamide is to flocculate solids in a very liquid. This method applies to water treatment, and processes like paper creating. The most consequence of this can be to present the ‘modified’ chemical compound a selected ionic character.
Another common use of polyacrylamide and its derivatives is in subterranean applications like increased Oil Recovery. High consistency liquid solutions is generated with low concentrations of polyacrylamide polymers, and these is injected to boost the social science of typical water flooding.
The anionic sort of cross-linked polyacrylamide is often used as a soil conditioner on farmland and construction sites for erosion management, so as to safeguard the water quality of close rivers.
The ionic sort of polyacrylamide has found a crucial role within the potable water treatment business. This permits water treatment plants to greatly improve the removal of total organic content (TOC) from raw water.
2.2.2 Environmental Effects of Polyacrylamide
Concerns are raised that polyacrylamide employed in agriculture could contaminate food with amide. Whereas polyacrylamide itself is comparatively non-toxic, it’s renowned that commercially obtainable polyacrylamide contains minute residual amounts of amide remaining from its production, typically but zero.05% w/w [2].
2.3 Reactive Dye
A dye that is capable of reacting with chemicals with a substrate to make a valence dye- substrate linkage is thought as a reactive dye.
Here the dye contains a reactive cluster and this reactive cluster makes the chemical bond with the fiber chemical compound and act as an integral a part of the fiber. This chemical bond is made between the dye molecules and therefore the terminal –OH (hydroxyl) cluster plastic fiber or between the dye molecules and therefore the terminal amino (- NH2) cluster of polymeric amide fibers.
The general formula of reactive dye will be written as following: D-X-Y
Here,
- D=Dye part (color producing part).
- X=Bridge.
- Y=Functional
D-X-Y+ Fiber = Fiber covalent bond
2.3.1 Properties of Reactive Dye
- Reactive dyes area unit anionic dyes that area unit used for coloring plastic super molecule polymer fibers.
- Reactive dyes area unit found in powder, liquid and print paste from.
- Throughout coloring, the reactive cluster of this dye forms a chemical bond with fiber compound and becomes an integral a part of the fiber.
- Reactive dyes area unit soluble in water. They need superb lightweight fastness with a rating of regarding half-dozen.
- The dyes have terribly stable lepton arrangement and also the degrading result ultraviolet ray.
- Reactive dyes provide brighter shads and have moderate rubbing fastness.
- Reactive dyes area unit relatively low-cost.
- Reactive dyes have smart perspiration fastness with rating 4-5.
- Fixation happens in alkaline condition.
2.3.2 Structure of Reactive Dyes
General structure of reactive dyes:
The general structure of reactive dye is: D-B-G-X. Chemical structure of reactive dyes:
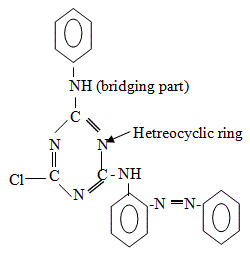
Here,
D= Dye part or chromogen (color producing part)
Dyes may be direct, acid, disperse, premetallised dye etc.
B = Bridging part.
Bridging part may be –NH- group or –NR- group.
G = Reactive group bearing part.
X= Reactive group [5].
2.3.3 Classification of Reactive Dyes
Reactive dyes can be classified in different classes which are given below:
1) Depends on Reactive Group:
- Halogen: Accumulation of nitrogen bearing heterocycle, like 3 types-
- Triazine group
- Pyridimine group
- Quinoxaline dyes
Example:
Triazine derivatives: procion, cibacron. Pyridimine derivatives: reactone Quinoxaline derivatives: levafix.
b) Activated vinyl compound:
- Vinyl sulphone
- Vinyl acrylamide
- Vinyl
Example:
Vinyl sulphone: remazol Vinyl acrylamide: primazine Vinyl sulphonamide: levafix.
2) Depends on Reactivity:
- Lower reactive dye: Medium reactive dye: By using Na2CO3, pH is controlled (11-12) on the dye
- Higher reactive dye: By using Na2CO3, pH is controlled (10-11) on the dye
3) Depends on Dyeing Temperature:
a) Cold brand:
Dyes contain reactive group which is higher reactivity. In lower temperature the dyeing is occurred i.e. 320-600 0C.
For example: PROCION M, LIVAFIX E.
b) Medium brand:
Dyes contain reactive group which is moderate reactivity. In higher temperature the dyeing is occurred compare to cold brand i.e. in between 600-710 0C temperatures. For example, Remazol, Livafix are medium brand dyes.
c) Hot brand:
Least reactivity group of reactive dye contain this brand. So it is necessary high temperature for dyeing i.e. 720-930 0C temperature is required for dyeing.
For example PRICION H, CIBACRON are hot brand dyes [1]
2.3.4 Functional Group of Reactive Dyes
Table 2.1: Functional group of reactive dye:
| Functions | Fixation | Temperature | Included in Brands |
| Monochlorotriazine | Haloheterocycle | 80°C | Basilen E & PCibacron EProcion H,HE |
| Monofluorochlorotriazine | Haloheterocycle | 40°C | Cibacron F & C |
| Dichlorotriazine | Haloheterocycle | 30°C | Basilen MProcion MX |
| Difluorochloropyrimidine | Haloheterocycle | 40°C | Levafix EADrimarene K & R |
| Dichloroquinoxaline | Haloheterocycle | 40°C | Levafix E |
| Trichloropyrimidine | Haloheterocycle | 80-98°C | Drimarene X & ZCibacron T |
| Vinyl sulfone | Activated double bond | 40°C | Remazol |
| Vinyl amide | Activated double bond | 40°C | Remazol |
2.3.5 Trade Names of Reactive Dye
Table 2.2: Trade name of reactive dye:
| Trade name | Manufacturer | Country |
| Procion | I.C.I | U.K |
| Ciba cron | Ciba | Switzerland |
| Remazol | Hoechst | Germany |
| Levafix | Bayer | Germany |
| Reactone | Geigy | Switzerland |
| Primazin | BASF | Germany |
| Drimarine | Sandoz | Switzerland |
2.3.6 Chemistry of Reactive Dyes
Reactive dyes disagree from different coloring matters in this they enter into reaction with fiber throughout coloring become a district of fiber substances. A reactive dye is diagrammatical as R-B-X, where, R Chromogenic, B-Bridging group, X-Reactive system. Once it reacts with fiber, F, it forms R-B-X-F. The wet fastness of unreal material made depends on the soundness of true covalent bond X-F [5].
2.3.7 Hydrolysis of Reactive Dye
Reactive dye has direct attraction for cellulose. It can be react with cellulose fiber (cotton) without the helping of any assistant.
Reactive dye makes covalent bond with cellulose
D-Y-F + OH-Cell—> D-Y-O-cell
Here,
D=Dye;
Y= Bridge;
F= Functional Group (Covalent Bond)
In alkaline condition, the affinity of reactive dye is increased because of improve their PH.
But when it keep in the alkaline condition for long time then reactive dye loses it utility. Because under Alkaline condition for long time, reactive dye reacts with water as a result reactive dye lost it working ability for increasing its PH.
D-Y-F + OH-H—> D-Y-OH
When reactive dye reacts with water and loses its utility then it called hydrolysis of reactive dye [3].
2.3.8 Important factors for Reactive Dyeing
- PH of the substrate prior to dyeing
- PH of the dye bath
- Pretreatment of the substrate
- Solubility of the dyestuff
- Dyeing temperature
- Quality of water and salt
- Electrolyte concentration
- Dyeing time
- Washing off sequence
2.3.9 Dyeing Mechanism of Reactive Dye
The coloring mechanism of cotton cloth with reactive dye takes place in three stages:
- Exhaustion of dye within the presence of electrolyte i.e. dyes
- Fixation is done by the influence of
- The unfixed dye remove from the fabric surface by wash-off.
2.3.10 Dye Exhaustion
When fiber is immersed in dye liquor, electrolyte is another to help the exhaustion of dye. Here NaCl is employed because the electrolyte. This electrolyte will increase the action of dyes. Therefore once the textile material is immersed into the dye liquor, the dye is exhausted on to the fiber.
2.3.11 Fixation
Dye fixation indicates the reaction of reactive group of dye with terminal –OH or-NH2- group of fiber and therefore make a covalent bond with the fiber. This can be a crucial part that is controlled by maintaining correct pH concentration by adding alkali. The alkali used for this creates correct pH concentration within the dye tub and work because the dye-fixing agent.
2.3.12 Wash-off
As the coloring is completed, an honest wash should be applied to the fabric to get rid of further and unfixed dyes from the fabric surface. This is often necessary for level coloring and sensible fastness properties. It’s done by a series of hot wash, cold wash and soap solution wash [6].
2.4 Cotton Fabric
Cotton could be a fiber that comes from the plant of the cotton and is employed to form several cloth varieties at each value purpose. The fiber is hollow within the center and, below the magnifier, resembles a twisted ribbon.

2.4.1 Physical Properties of Cotton
Physical properties of cotton fibers square measure given below:
- Color: The color of cotton fiber may well be white, creamy white, bluish-white, yellowish-white or grey.
- Tensile Strength: Cotton is moderately sturdy fiber. The determination of 3-5 gm/den. The wet strength of cotton is 20%.
- Elongation at break: It’s associate degree elongation at break of 5-10%.
- Elastic Recovery: At 2% extension it’s associate degree ER of 74% associate degree at 5% extension it’s an ER of 45%.
- Specific Gravity: Relative density is 1.54
- Moisture Regain (MR %): Commonplace wetness regain is 8.5
- Effect of Heat: Cotton has a superb resistance to degradation by heat. It begins to show yellow when many hours at 12000C and decomposes marked by at 15000 As a results of oxidization, cotton is severally broken when couple of minutes at 24000C.
- Effect of Sun Light: There’s a gradual loss of strength once cotton is exposed to sun light-weight and also the fiber turns yellow. By sun light-weight abundant of the harm is caused by UV-light and by the shorten weaves of visible radiation.
- Moisture Content (MC %): Commonplace wetness content is 7. 834.
2.4.2 Chemical Properties of Cotton
Chemical properties of the cotton fiber are given below:
- Effect of Acids: Cotton is attacked by hot dilute acids or cold focused acids that it disintegrates. It’s not full of acids.
- Effects of Alkalis: Cotton has a wonderful resistance to alkalis. It swells in caustic alkalis (NaOH) however it doesn’t harm. It may be washed repeatedly in soap answer with none downside.
- Effect of Organic Solvent: Cotton has high resistance to traditional cleanup solvents. Cotton is dissolved by the copper complexes, like cuprammonium hydroxide, cupriethylene organic compound and focused 70% H2SO4
- Effects of Insects: Cotton isn’t attacked by moth-grubs or beetles.
- Effect of small Organism: Cotton is attacked by fungi and bacterium [1].
EXPERIMENTAL DETAILS
3.1 Materials
In this research, we used three knit grey fabric samples. The samples specification are given below in table 3.1
Table 3.1: Samples Specification before Dyeing
| Sample No. | Type of Fabric | GSM (gm/m2) | WPI | CPI | Stitch Length (in mm) |
| 01 | Single Jersey | 141 | 35 | 53 | 2.45 |
| 02 | (2×2) Rib | 246 | 51 | 45 | 3.41 |
| 03 | Plain Interlock | 223 | 66 | 78 | 3.95 |
3.1.1 Chemicals Used
Chemicals Used for Scouring & Bleaching:
- Detergent
- Hydrogen Peroxide (H2O2)
- Peroxide Stabilizer
- Sequestering Agent
- Sodium Hydroxide (NaOH)
Table No. 3.2 The chemicals used in scouring and bleaching process and their function.
| Chemical | Function |
| Detergent |
|
| Hydrogen Peroxide (H2O2) |
|
| Peroxide Stabilizer |
|
| Sequestering Agent |
|
| Sodium Hydroxide (NaOH) |
|
3.1.2 Method of Scouring and Bleaching
At first we collected the following three types of sample. Treated with the addition of Detergent, Hydrogen Peroxide (H2O2), Peroxide Stabilizer, Sequestering Agent, Sodium Hydroxide (NaOH) and required amount of water at 1000C for 60 minutes. We were maintained the time, temperature and pH according to scouring and bleaching process recipe. The recipe of the process is mentioned in table 3.3
Table 3.3: Scouring and Bleaching Recipe
| Particulars | Parameters of the Recipe |
| Detergent | 1 g/l |
| Hydrogen Peroxide (H2O2) | 5 g/l |
| Peroxide Stabilizer | 2 g/l |
| Sequestering Agent | 1 g/l |
| Sodium Hydroxide (NaOH) | 1 g/l |
| Sample Weight | 90 gm (30 gm S/J, 30 gm Rib and 30 gm Plain Interlock) |
| M:L | 1:40 |
| pH | (5-6) |
| Temperature | 1000C |
| Time | 60 minutes |
3.1.3 Apparatus Used
- Electric Balance
- Scissors
- Beaker
- Glass Rod
- Pot
- Pipette
- pH Meter
- Thermometer
- Gas Burner
- Tripod Stand
3.1.4 Calculation
Recipe calculation of scouring and bleaching:
Total Liquor = 90×40 = 3600 ml
Detergent = (1 X 3600) ÷ 1000 ml = 3.6 ml
Hydrogen Peroxide (H2O2) = (5 X 3600) ÷ 1000 ml = 18 ml
Peroxide Stabilizer = (2 X 3600) ÷ 1000 ml = 7.2 ml
Sequestering Agent = (1 X 3600) ÷ 1000 ml = 3.6 ml
Sodium Hydroxide (NaOH) = (1 X 3600) ÷ 1000 ml = 3.6 gm
⸫ Required amount of water = {3600 – (3.6+18+7.2+3.6)} ml
= (3600 – 32.4) ml
= 3567.8 ml
3.1.5 Process Sequence of Scouring and Bleaching
Gathering of 100% cotton knit fabric
↓
Add chemicals with required amount of water
↓
Sum up the sample in the bath
↓
Treated at 1000C for 60 minutes
↓
Bath drop
↓
Cold rinsing
↓
Drying
3.1.6 Process Curve of the Scouring and Bleaching
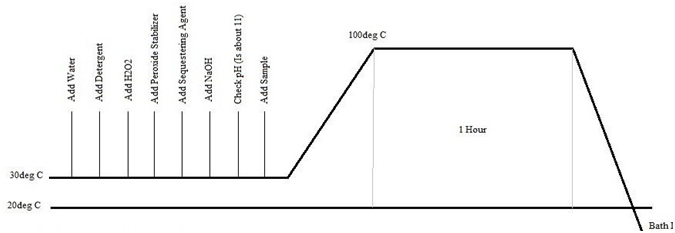
3.1.7 Working Procedure of Scouring and Bleaching
- At first, we setup the dye bath above the tripod stand.
- Then accumulate all the chemicals and required amount of water onto the day bath.
- After that, took the sample and put onto the bath.
- After the following stapes, we control the pH, increase the temperature at up to1000C for 60 minutes.
- When the following process is completed, the bath set down then the sample wash with cold water.
- Finally, the sample drying in the open air.
3.2 Fiber Modification
3.2.1 Chemical Used for Fiber Modification
- Polyacrylamide
- Wetting Agent
Table 3.4: Function of the using chemicals.
| Chemicals Name | Functions |
| Polyacrylamide |
|
| Wetting Agent |
|
Table 3.5: Recipe of Fiber Modification Treatment
| Particulars | Recipe Parameters |
| Polyacrylamide | 5%, 10%, 15%, 20%, 25% (owf) |
| Wetting agent | 1 g/l |
| Sample weight | 18 gm (S/J = 6 gm + Rib = 6 gm + Interlock = 6 gm) |
| M:L | 1:30 |
| pH | (7-8) |
| Temperature | Normal (30 0C) |
| Time | 30 Minutes |
| Curing Temperature | 120 0C |
| Curing Time | 7 Minutes |
3.2.2 Apparatus Used
- Electric Balance
- Scissors
- Scale
- Glass Rod
- Pot
- Pipette
- pH Meter
3.2.3 Calculation
Recipe Calculation of Fiber Modification:
Total Liquor = 18 X 30 = 540 ml
Polyacrylamide,
For 5% = (5 X 18) ÷ 100 gm = 0.9 gm
For 10% = (10 X 18) ÷ 100 gm = 1.8 gm
For 15% = (15 X 18) ÷ 100 gm = 2.7 gm
For 20% = (20 X 18) ÷ 100 gm = 3.6 gm
For 25% = (25 X 18) ÷ 100 gm = 4.5 gm
Wetting Agent = (1 X 540) ÷ 1000 ml = 0.54 ml
⸫ Required amount of water = (540 – 0.54) ml
= 539.46 ml
3.2.4 Process Sequence of Fiber Modification
Culled of the Treated Samples
↓
Cut the Samples into 5 Pieces
↓
Adopt 5 pots and put those samples into those 5 pots
↓
Accumulate the required amount of water & chemicals into 5 pots
↓
Stir the samples and mixed with those chemicals and water
↓
Stir the samples at room temperature for 30 minutes
↓
Bath drop and squeeze the samples
↓
Curing at 1200C for 7 minutes
3.2.5 Working Procedure
- First of all, we gathering our treated samples and pots and cut the samples into 5 pieces.
- Then put the samples onto the 5 pots.
- After that, affix required amount of water and chemicals onto those pots.
- Mixed the samples with the composition.
- Stir the samples with the mixture at room temperature for 30 minutes.
- After treatment, we put down the pots and squeeze the samples.
- At last, we curing those samples at 1200C for 7 minutes.
3.3 Dyeing
3.3.1 Chemical Used for Dyeing Process-
- Reactive dye
- Wetting agent
- Levelling agent
Table 3.6: Function of the using chemicals.
| Chemicals Name | Functions |
| Reactive dye | Used to color the fabric. |
| Wetting agent | Used to minimize the surface tension of water. |
| Levelling agent | Used to uniform dyeing. |
Table 3.7: Recipe of Dyeing
| Particulars | Recipe Parameters |
| Reactive dye | 2% owf |
| Wetting agent | 1 g/l |
| Levelling agent | 1 g/l |
| Sample weight | 18 gm (S/J = 6 gm + Rib = 6 gm + Interlock = 6 gm) |
| M:L | 1:30 |
| pH | (10-11) |
| Temperature | 60 0C |
| Time | 30 Minutes |
3.3.2 Apparatus Used
- Electric Balance
- Scissors
- Beaker
- Glass Rod
- Pot
- Pipette
- pH Meter
- Thermometer
- Gas Burner
- Tripod Stand
3.3.3 Calculation
Recipe Calculation of Dyeing:
Total Liquor = 18 X 30 = 540 ml
Reactive dye = (2 X 18) ÷ 100 gm = 0.36 gm
Wetting Agent = (1 X 540) ÷ 1000 ml = 0.54 ml
Levelling agent = (1 X 540) ÷ 1000 ml = 0.54 ml
⸫ Required amount of water = {540 – (0.54 + 0.54)} ml
= 538.92 ml
3.3.4 Process Sequence of Dyeing
Culled of the modified samples
↓
Accumulate the required amount of water & chemicals into 5 pots
↓
Adopt 5 pots and put those samples into those 5 pots
↓
Stir the samples and mixed with those chemicals and water
↓
Increase the temperature at 600C for 30 minutes
↓
Bath drop and cold wash the samples
↓
Drying in the open air
3.3.5 Process Curve of Dying
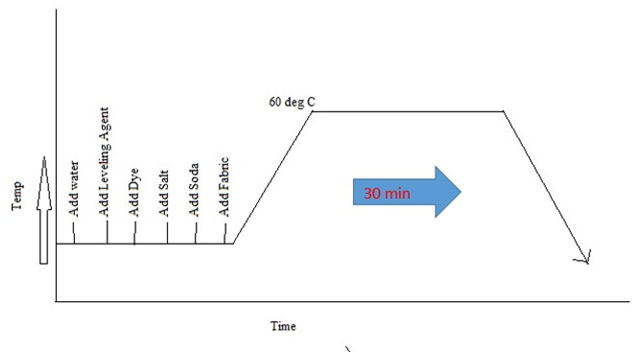
3.3.6 Procedure of Dyeing
- First of all, we gathering 5 pots and divided our modified samples into those pots.
- Affix required amount of water and chemicals into those pots, rise temperature at 600C dyeing for 30 minutes.
- After dyeing we put down the dye bath, wash the samples with cold water.
- Finally we dry those samples in the open air.
3.4 Procedure of Evaluation
3.4.1 Measurement of WPI & CPI
In the knit cloth the yarn is measured in horizontal and vertical direction which we commonly known as course and wales direction. When the yarn is in course per inch then it indicates CPI and when the yarn is in wales per inch then it indicates WPI.
- Take the material and marking (1 x 1) inch with the ball pen in line with the course and wales wise of a cloth.
- Then set the marking purpose with the number scale and investigating the CPI and WPI of cloth in 1 inch.
- Wales per inch and course per inch are unit counted by the counting glass of magnifying.

3.4.2 Measurement of GSM
- Holding the sample.
- By using the scissors cut the sample into (1 inch X 1 inch).
- By using electric balance take the weight of the cutting sample.
- Find the weight in gm/in2.
- Finally changing the weight in gm/m2.

3.4.3 Measurement Stitch Length
Firstly 100 number of wales we selected or counted and indicate the ends of all wales.
After that, we uncovered one by one yarn and measured the length (in mm) of all uncovered yarn which we indicated.
By using the following formula the stitch length is measured,
![]()

3.5 Color Fastness to Wash
3.5.1 Chemical Used for Color Fastness to Wash
- Detergent
- Soda / Sodium Carbonate Ash
Table 3.8: Function of the using chemicals.
| Chemicals Name | Functions |
| Detergent | Used to take aside the impurities of fabric and the extra color. |
| Soda Ash / Sodium Carbonate | It has the power of cleaning and used to help the effect of color fading of fabric. |
Table 3.9: Recipe of Washing (ISO-105-CO3)
| Particulars | Recipe Parameters |
| Detergent | 5 g/l |
| Soda Ash / Sodium Carbonate | 2 g/l |
| M:L | 1:50 |
| Temperature | 60 0C |
| Time | 30 Minutes |
3.5.2 Apparatus Used
- Beaker.
- Measuring Cylinder.
- Pipette.
- Pot.
- Digital Balance.
- Scissor.
- Tri-pod stand.
- Gas Burner.
- Thermometer.
- Gray scale for color change.
- Gray scale for color fading.
- Light Box.
- Dryer.
- Multi fiber.
3.5.3 Sample Size
Take a sample and cut it into 10 cm x 4 cm and sewing it with the multi-fiber fabric in same size. It is the test sample.
3.5.4 Calculation
Recipe Calculation of Washing:
Total Liquor = 500 ml
Detergent = (5 X 500) ÷ 1000 ml = 2.5 ml
Soda Ash / Sodium Carbonate = (2 X 500) ÷ 1000 gm = 1 gm
⸫ Required amount of water = (500 – 2.5) ml
= 497.5 ml
3.5.5 Process Sequence of Washing
Sample & Multi-fiber conditioned as workplace of lab standard (20±2) °C temperature and relative humidity (65±2).
↓
Gathering multi-fiber & sample of 10X4 cm2.
↓
Sewing each multi-fiber and take a look at specimen along.
↓
Wash seamed specimen as per direction.
↓
Final assessment with each sort of greyscale.
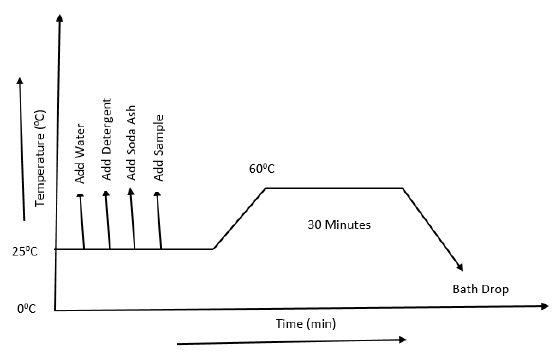
3.5.6 Process Curve of Washing
3.5.7 Working Procedure
- At first, we like to gather the conditioned bleached sample and multi-fiber of 10X4 cm2 and sew them along.
- Then we like to line a shower and take chemicals as per formula.
- Then we tend to immersed sample to the tub and heated it up to 60°C for half- hour.
- When the method, it must dry the sample and compare with grayscale to color modification for the bleached sample and compare with grayscale to paint staining for multi-fiber.
3.6 Color Fastness to Rubbing
3.6.1 Apparatus Used
- Crock meter
- Cotton rubbing cloth
- Grey Scale
- Stop watch and
- Color matching cabinet.
3.6.2 Size of Fabric
14 cm X 5 cm two pieces of sample (one warp direction/ wale direction and other weft/ course direction).
3.6.3 Test Procedure
- Lock the check specimen onto the bottom of the crock meter.
- Using the spinal spring clip, set five cm X five cm of the white cotton cloth to the finger of the crock meter.
- Lower the coated finger on the check sample.
- Turn hand crank at the speed of 1 flip per second (10 x ten sec).
- Remove the white rubbing check material and assess with greyscale.
3.4.6 Evaluation
- Compare the distinction between the treated and untreated white rubbing material with greyscale and rated 1-5
SAMPLE ATTACHMENT
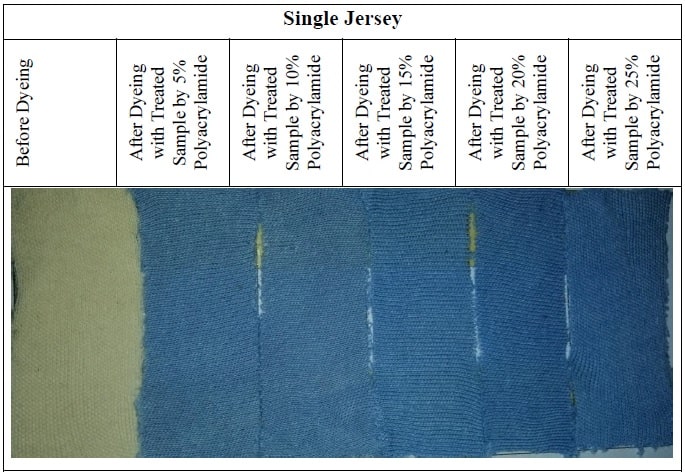
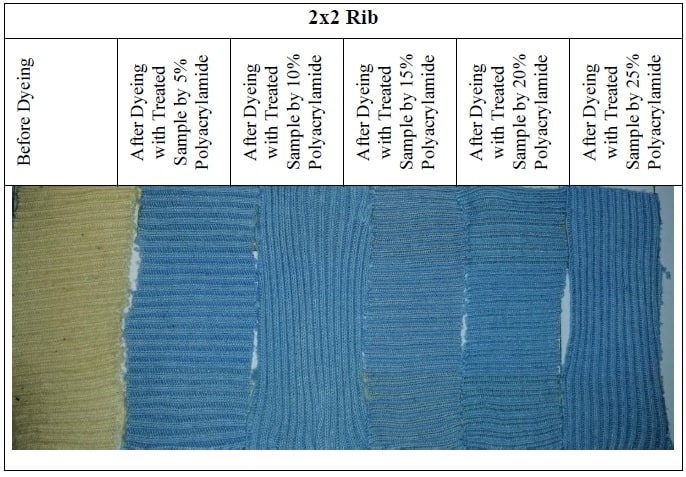
3.7 Sample Attachment for Before and After Dyeing
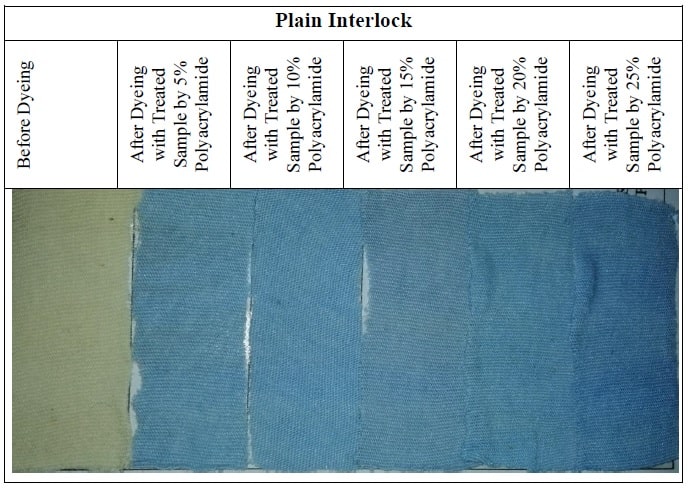
DISCUSSION OF RESULTS/FINDINGS
Table 4.1 Change in GSM of Cotton fabrics After Dyeing Process
| Sample No. | Types of Fabric | Conc. Of Polyacrylamide (%) | GSM of Fabric Before Dyeing (gm/m2) | GSM of Fabric After Dyeing (gm/m2 ) | Change of GSM (gm/m2 ) | % of Change of GSM |
| 1 | Single Jersey | 5 | 141 | 142 | 1 | 0.70% |
| 2 | 10 | 143 | 2 | 1.42% | ||
| 3 | 15 | 143 | 2 | 1.42% | ||
| 4 | 20 | 144 | 3 | 2.13% | ||
| 5 | 25 | 145 | 4 | 2.84% | ||
| 6 | 2×2 Rib | 5 | 246 | 248 | 2 | 0.81% |
| 7 | 10 | 250 | 4 | 1.62% | ||
| 8 | 15 | 251 | 5 | 2.03% | ||
| 9 | 20 | 252 | 6 | 2.44% | ||
| 10 | 25 | 253 | 7 | 2.48% | ||
| 11 | Plain Interlock | 5 | 225 | 226 | 1 | 0.44% |
| 12 | 10 | 228 | 3 | 1.33% | ||
| 13 | 15 | 229 | 4 | 1.78% | ||
| 14 | 20 | 231 | 6 | 2.67% | ||
| 15 | 25 | 233 | 8 | 3.56% |
Here, the table no. 4.1 indicates the amendment of GSM. The table indicates % of the amendment in GSM for a distinct amendment of amendment in GSM for various varieties of fabric. In Reactive dye, we will see that the GSM of single jersey material is 141 before coloring. However we tend to get that GSM when coloring constant material in 5 segments area unit 142, 143, 143, 144 and 145. Therefore we tend to get the amendment of GSM area unit 1, 2, 2, 3 and 4 at the same time. And also the percentage of amendment in GSM area unit 0.70, 1.42, 1.42, 2.13 and 2.84 at the same time.
In Reactive dye, we are able to see that, the GSM of 2×2 Rib material is 246 before coloring. However we tend to get that GSM once coloring identical material in 5 segments area unit 248, 250, 251, 252 and 253. Therefore we tend to get the modification of GSM area unit are 2, 4, 5, 6 and 7 at the same time. And also the Percentage of modification in GSM area unit 0.81, 1.62, 2.03, 2.44 and 2.48 at the same time.
In Reactive dye, we will see that, the GSM of plain interlock cloth is 225 before coloring. However we have a tendency to get that GSM when coloring identical cloth in 5 segments 226, 228, 219, 231 and 233. Thus we have a tendency to get the modification of GSM are 1, 3, 4, 6 and 8 at the same time. And also the Percentage of modification in GSM are 0.44, 1.33, 1.78, 2.67 and 3.56 at the same time.
Table 4.2 Change in WPI of Cotton Fabrics after Dyeing Process
| Sample No. | Types of Fabric | Conc. Of Polyacrylamide (%) | WPI of Fabrics Before Dyeing | WPI of Fabrics After Dyeing | Change of WPI | % of Change of WPI |
| 1 | Single Jersey | 5 | 35 | 37 | 3 | 8.57% |
| 2 | 10 | 39 | 4 | 11.42% | ||
| 3 | 15 | 40 | 5 | 14.28% | ||
| 4 | 20 | 42 | 7 | 20.00% | ||
| 5 | 25 | 43 | 8 | 22.85% | ||
| 6 | 2×2 Rib | 5 | 51 | 52 | 1 | 1.96% |
| 7 | 10 | 54 | 3 | 5.88% | ||
| 8 | 15 | 55 | 4 | 7.84% |
| 9 | 20 | 57 | 6 | 11.76% | ||
| 10 | 25 | 58 | 7 | 13.72% | ||
| 11 | Plain Interlock | 5 | 66 | 68 | 2 | 3.03% |
| 12 | 10 | 69 | 3 | 4.55% | ||
| 13 | 15 | 71 | 5 | 7.57% | ||
| 14 | 20 | 72 | 6 | 9.09% | ||
| 15 | 25 | 74 | 8 | 12.12% |
Here, the table no. 4.2 indicates the amendment of WPI. The table indicates % of the amendment in WPI for a distinct amendment of amendment in WPI for various varieties of fabric. In Reactive dye, we will see that the WPI of single jersey material is 35 before coloring. However we tend to get that WPI when coloring constant material in 5 segments area unit 37, 39, 40, 42 and 43. Therefore we tend to get the amendment of WPI area unit 3, 4, 5, 7 and 8 at the same time. And also the percentage of amendment in WPI area unit 8.57, 11.42, 14.28, 20.00 and 22.85 at the same time.
In Reactive dye, we are able to see that, the WPI of 2×2 Rib material is 51 before coloring. However we tend to get that WPI once coloring identical material in 5 segments area unit 52, 54, 55, 57 and 58. Therefore we tend to get the modification of WPI area unit are 1, 3, 4, 6 and 7 at the same time. And also the Percentage of modification in WPI area unit 1.96, 5.88, 7.84, 11.76 and 13.72 at the same time.
In Reactive dye, we will see that, the WPI of plain interlock cloth is 66 before coloring. However we have a tendency to get that WPI when coloring identical cloth in 5 segments 68, 69, 71, 72 and 74. Thus we have a tendency to get the modification of WPI are 2, 3, 5, 6 and 8 at the same time. And also the Percentage of modification in WPI are 3.03, 4.55, 7.57, 9.09 and 12.12 at the same time.
Table 4.3 Change in CPI of Cotton Fabrics after Dyeing Process
| Sample No. | Types of Fabric | Conc. Of Polyacrylamide (%) | CPI of Fabrics Before Dyeing | CPI of Fabrics After Dyeing | Change of CPI | % of Change of CPI |
| 1 | Single Jersey | 5 | 56 | 58 | 2 | 3.57% |
| 2 | 10 | 60 | 4 | 7.14% | ||
| 3 | 15 | 61 | 5 | 8.92% | ||
| 4 | 20 | 63 | 7 | 12.50% | ||
| 5 | 25 | 64 | 8 | 14.28% | ||
| 6 | 2×2 Rib | 5 | 45 | 47 | 2 | 4.44% |
| 7 | 10 | 49 | 4 | 4.89% | ||
| 8 | 15 | 50 | 5 | 11.11% | ||
| 9 | 20 | 52 | 7 | 15.16% | ||
| 10 | 25 | 54 | 9 | 20.00% | ||
| 11 | Plain Interlock | 5 | 78 | 80 | 2 | 2.56% |
| 12 | 10 | 81 | 3 | 3.84% | ||
| 13 | 15 | 83 | 5 | 6.41% | ||
| 14 | 20 | 84 | 6 | 7.69% | ||
| 15 | 25 | 86 | 8 | 10.26% |
Here, the table no. 4.3 indicates the amendment of CPI. The table indicates % of the amendment in CPI for a distinct amendment of amendment in CPI for various varieties of fabric. In Reactive dye, we will see that the CPI of single jersey material is 56 before coloring. However we tend to get that CPI when coloring constant material in 5 segments area unit 58, 60, 61, 63 and 64. Therefore we tend to get the amendment of CPI area unit 2, 4, 5, 7 and 8 at the same time. And also the percentage of amendment in CPI area unit 3.57, 7.14, 8.92, 12.50 and 14.28 at the same time.
In Reactive dye, we are able to see that, the CPI of 2×2 Rib material is 45 before coloring. However we tend to get that CPI once coloring identical material in 5 segments area unit 47, 49, 50, 52 and 54. Therefore we tend to get the modification of CPI area unit are 2, 4, 5, 7 and 9 at the same time. And also the Percentage of modification in CPI area unit 4.44, 4.89, 11.11, 15.16 and 20.00 at the same time.
In Reactive dye, we will see that, the CPI of plain interlock cloth is 78 before coloring. However we have a tendency to get that CPI when coloring identical cloth in 5 segments 80, 81, 83, 84 and 86. Thus we have a tendency to get the modification of CPI are 2, 3, 5, 6 and 8 at the same time. And also the Percentage of modification in CPI are 2.56, 3.84, 6.41, 7.69 and 10.26 at the same time.
Table 4.4 Change in Stitch Length of Cotton Fabrics after Dyeing Process
| Sample No. | Types of Fabric | Conc. Of Polyacrylamide (%) | Stitch Length of Fabrics Before Dyeing (mm) | Stitch Length of Fabrics After Dyeing (mm) | Change of Stitch Length (mm) | % of Change of Stitch Length (mm) |
| 1 | Single Jersey | 5 | 2.45 | 2.97 | 0.52 | 21.22% |
| 2 | 10 | 3.02 | 0.57 | 23.26% | ||
| 3 | 15 | 3.07 | 0.62 | 25.30% | ||
| 4 | 20 | 3.08 | 0.63 | 25.71% | ||
| 5 | 25 | 3.09 | 0.64 | 26.12% | ||
| 6 | 2×2 Rib | 5 | 3.40 | 3.82 | 0.42 | 12.35% |
| 7 | 10 | 3.85 | 0.45 | 13.24% | ||
| 8 | 15 | 3.87 | 0.47 | 13.82% | ||
| 9 | 20 | 3.89 | 0.49 | 14.41% |
| 10 | 25 | 3.92 | 0.52 | 15.29% | ||
| 11 | Plain Interlock | 5 | 3.95 | 4.12 | 0.17 | 4.30% |
| 12 | 10 | 4.15 | 0.20 | 5.06% | ||
| 13 | 15 | 4.18 | 0.23 | 5.82% | ||
| 14 | 20 | 4.21 | 0.26 | 6.58% | ||
| 15 | 25 | 4.23 | 0.28 | 7.08% |
Here, the table no. 4.4 indicates the amendment of Stitch Length. The table indicates % of the amendment in Stitch Length for a distinct amendment of amendment in Stitch Length for various varieties of fabric. In Reactive dye, we will see that the Stitch Length of single jersey material is 2.45 mm before coloring. However we tend to get that Stitch Length when coloring constant material in 5 segments area unit 2.97, 3.02, 3.07, 3.08 and 3.9 mm. Therefore we tend to get the amendment of Stitch Length area unit 0.52, 0.57, 0.62, 0.63 and 0.64 at the same time. And also the percentage of amendment in Stitch Length area unit 21.22, 23.26, 25.30, 25.71 and 26.12 at the same time.
In Reactive dye, we are able to see that, the Stitch Length of 2×2 Rib material is 3.40 mm before coloring. However we tend to get that Stitch Length once coloring identical material in 5 segments area unit 3.82, 3.85, 3.87, 3.89 and 3.92 mm. Therefore we tend to get the modification of Stitch Length area unit are 0.42, 0.45, 0.47, 0.49 and 0.52 mm at the same time. And also the Percentage of modification in Stitch Length area unit 12.35, 13.24, 13.82, 14.41 and 15.29 at the same time.
In Reactive dye, we will see that, the Stitch Length of plain interlock cloth is 3.95 mm before coloring. However we have a tendency to get that Stitch Length when coloring identical cloth in 5 segments 4.12, 4.15, 4.18, 4.21 and 4.23 mm. Thus we have a tendency to get the modification of Stitch Length are 0.17, 0.20, 0.23, 0.26 and 0.28 mm at the same time. And also the Percentage of modification in Stitch Length are 4.30, 5.06, 5.82, 6.58 and 7.08 at the same time.
Table 4.5 Color Fastness to Rubbing in Staining Scale
| SL No. | Concentration of Polyacrylamide | Sample Type | Rating for Dry Rubbing | Meaning | Rating for Wet Rubbing | Meaning |
|
1 |
5 | Single Jersey | 4-5 | Good to Excellent | 4 | Good |
| (2×2) Rib | 4-5 | Good to Excellent | 4 | Good | ||
| Plain Interlock | 4-5 | Good to Excellent | 4 | Good | ||
|
2 |
10 | Single Jersey | 4-5 | Good to Excellent | 4 | Good |
| (2×2) Rib) | 4-5 | Good to Excellent | 4 | Good | ||
| Plain Interlock | 4-5 | Good to Excellent | 4 | Good | ||
|
3 |
15 | Single Jersey | 4-5 | Good to Excellent | 4 | Good |
| (2×2) Rib | 4-5 | Good to Excellent | 4 | Good | ||
| Plain Interlock | 4-5 | Good to Excellent | 4 | Good | ||
|
4 |
20 | Single Jersey | 5 | Excellent | 4-5 | Good to Excellent |
| (2×2) Rib | 5 | Excellent | 4-5 | Good to Excellent | ||
| Plain Interlock | 5 | Excellent | 4-5 | Good to Excellent | ||
|
5 |
25 | Single Jersey | 5 | Excellent | 4-5 | Good to Excellent |
| (2×2) Rib | 5 | Excellent | 4-5 | Good to Excellent | ||
| Plain Interlock | 5 | Excellent | 4-5 | Good to Excellent |
From the following table we can see,
The three types of fabric which we are used for color fastness to rubbing purpose, when we used (5%, 10%, 15%) concentration of Polyacrylamide we get the ratting in staining scale are (4-5) means Good to Excellent for dry condition and 4 means Good for wet condition.
On the other hand, when we used (20% & 25%) concentration of Polyacrylamide on those fabrics we find the ratting in dry condition 5 means Excellent and in wet condition (4-5) means Good to Excellent.
In the instance of color fastness to rubbing, the very best decrease in wet rubbing is found 4 for single jersey, 2×2 rib & Plain Interlock when reactive dyeing with (5%, 10% & 15%) concentration of Polyacrylamide. and also the highest increase in wet rubbing is found 4-5 for all fabrics when reactive dyeing with (20% & 25%) concentration of Polyacrylamide. The highest increase in dry rubbing is found 5 in single jersey, 2×2 rib & Plain Interlock when reactive dyeing with (20% & 25%) concentration of Polyacrylamide. and also the highest decrease in dry rubbing is found 4-5 for all fabrics when reactive dyeing with ((5%, 10% & 15%) concentration of Polyacrylamide.
Table 4.6 Color Fastness to Washing in Color Change
| SI No. | Concentration Of Polyacrylamide (%) | Sample Type | Multi-Fiber Fabric | |||||
| Acetate | Cotton | Nylon | Polyester | Acrylic | Wool | |||
| 1 | 5 | Single Jersey | 4-5 | 4 | 4-5 | 4-5 | 4-5 | 4-5 |
| (2×2) Rib | 4-5 | 4 | 4-5 | 4-5 | 4-5 | 4-5 | ||
| Plain Interlock | 4-5 | 4 | 4-5 | 4-5 | 4-5 | 4-5 | ||
| 2 | 10 | Single Jersey | 5 | 4 | 5 | 5 | 4-5 | 4-5 |
| (2×2) Rib | 5 | 4 | 5 | 5 | 4-5 | 4-5 | ||
| Plain Interlock | 5 | 4 | 5 | 5 | 4-5 | 4-5 | ||
| 3 | 15 | Single Jersey | 5 | 4-5 | 5 | 5 | 5 | 5 |
| (2×2) Rib | 5 | 4-5 | 5 | 5 | 5 | 5 | ||
| Plain Interlock | 5 | 4-5 | 5 | 5 | 5 | 5 | ||
| 4 | 20 | Single Jersey | 5 | 4-5 | 5 | 5 | 5 | 5 |
| (2×2) Rib | 5 | 4-5 | 5 | 5 | 5 | 5 | ||
| Plain Interlock | 5 | 4-5 | 5 | 5 | 5 | 5 | ||
| 5 | 25 | Single Jersey | 5 | 4-5 | 5 | 5 | 5 | 5 |
| (2×2) Rib | 5 | 4-5 | 5 | 5 | 5 | 5 | ||
| Plain Interlock | 5 | 4-5 | 5 | 5 | 5 | 5 |
According to the following table 4.6 we can see,
Different concentration of Polyacrylamide effect on the fibers of the fabrics which we are applied for color fastness to washing. That’s mean when we used 5% concentration of Polyacrylamide the ratting of multi-fiber fabric is between (4-5) except cotton fiber for which we get the rating of cotton 4.
Same as when we used 10% concentration of Polyacrylamide we find the ratting for Acetate, Nylon, Polyester 5; Acrylic & Wool for (4-5) and Cotton for 4.
For the using of 15%, 20% & 25% concentration of Polyacrylamide we get the ratting of multi-fiber fabric is between 5 except cotton fiber for which we get the ratting for cotton (4-5).
In the instance of color fastness to washing, the best increase in color modification is found 5 in single jersey, (2×2) rib & Plain Interlock when reactive dyeing with (15%, 20% & 25%) concentration of Polyacrylamide. and therefore the highest decrease in color modification is found 4-5 in single jersey, 2×2 rib and interlock when reactive dyeing with (5%, & 10%) concentration of Polyacrylamide.
CONCLUSION
The purpose of this paper is to allow an overall plan regarding the pre-treatment of cotton with a Polyacrylamide may enhance the dye ability of the fiber with reactive dyes. After completing the project, we have learnt about the effect of different concentration of Polyacrylamide used for dyeing of cotton fabric with reactive dye. Finally we gain the following outcomes-
- In instance of plain interlock fabric, the highest GSM is found 3.56% after treated with 25% concentration of Polyacrylamide and the lowest GSM is found 0.44% in 5% Polyacrylamide treatment.
- In condition of single jersey fabric, the highest increase of WPI is found 22.85% after treated with 25% Polyacrylamide, on the other hand, the lowest amount of WPI (1.96%) is getting from 2×2 rib fabric when treated with 5% Polyacrylamide. In situation of single 2×2 Rib fabric, the highest increase of CPI is found 20.00% after treated with 25% Polyacrylamide, on the other hand, the lowest amount of CPI (2.56%) is getting from Plain Interlock fabric when treated with 5% Polyacrylamide.
- In an instance of single jersey fabric, the highest increase of Stitch Length is found 26.12% after treated with 25% Polyacrylamide, on the other hand, the lowest amount of Stitch Length (4.30%) is getting from Plain Interlock fabric when treated with 5% Polyacrylamide.
- In the instance of color fastness to rubbing, the very best decrease in wet rubbing is found 4 in single jersey, 2×2 rib & Plain Interlock when reactive dyeing with (5%, 10% & 15%) concentration of Polyacrylamide. and also the highest increase in wet rubbing is found 4-5 in all fabrics when reactive dyeing with (20% & 25%) concentration of Polyacrylamide.
- The highest increase in dry rubbing is found 5 in single jersey, 2×2 rib & Plain Interlock when reactive dyeing with (20% & 25%) concentration of Polyacrylamide. and also the highest decrease in dry rubbing is found 4-5 in all fabrics when reactive dyeing with ((5%, 10% & 15%) concentration of Polyacrylamide.
- In the instance of color fastness to washing, the best increase in color modification is found 5 in single jersey, (2×2) rib & Plain Interlock when reactive coloring with (15%, 20% & 25%) concentration of Polyacrylamide. and therefore the highest decrease in color modification is found 4-5 in single jersey, 2×2 rib and interlock when reactive coloring with (5%, & 10%) concentration of Polyacrylamide.
For the using of polyacrylamide the environmental effect is eco-friendly and its comparatively cheap than using of salt and alkali on cotton fabric dyeing with reactive dye.
REFERENCES:
- https://textilelearner.net/salt-and-alkali-free-reactive-dyeing-on-cotton-fabric/ (Retrieved date: 03.02.2019, Retrieved time: 09:10 PM)
- Lecture sheet of Sumon Mozumder (Retrieved date: 25.02.2019, Retrieved time: 09:30 PM)
- https://www.fibre2fashion.com/industry-article/1661/salt–alkali-free-reactive (Retrieved date: 05.03.2019, Retrieved time: 10:00 PM)
- https://textilelearner.net/reactive-dyes-classification-dyeing-mechanism/ (Retrieved date: 25.03.2019, Retrieved time: 11:10 PM)
- https://www.slideshare.net/AlHafijProdhan/alkali-salt-free-dyeing-of-cotton-fabric-with-reactive-dye (Retrieved date: 09.04.2019, Retrieved time: 80:10 PM)
- https://en.wikipedia.org/wiki/Cotton (Retrieved date: 18.04.2019, Retrieved time: 10:10 PM)
